AI based prediction of drug side effects

Combination of knowledge graphs and data within AI algorithms
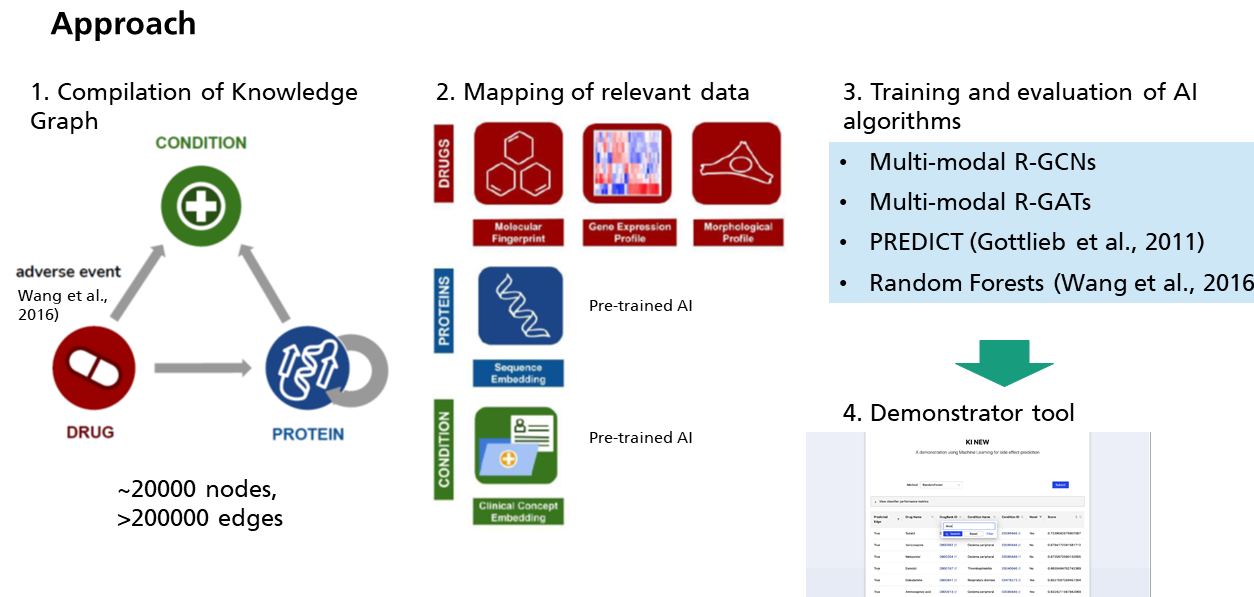
Initially, a big knowledge graph (~20,000 nodes, >200,000 edges) was compiled in order to represent literature known relationships between compounds, proteins, indication areas and side effects (subsumed as “conditions”). This graph was enriched with additional data, namely representations of medical concepts and protein sequences, molecular fingerprints, gene expression profiles and image-based descriptors of cell morphological changes. Based on the complex combination of a knowledge graph plus multi-modal data several AI algorithms were trained and evaluated. This comparison clearly demonstrated the feasibility of the envisioned project goal. Subsequently, a web based demonstrator tool was developed, which enables to use AI based predictions.
High economic potential: Minimizing of risks in advance
Unwanted side effects are one of the major reasons for the failure of clinical trials during drug development, which can result into massive damage of a company’s image. Hence, there is a high interest from industry in approaches, which can minimize according risks in advance. AI based approaches could in the future be an important and comparably cost-efficient solution.
AI approaches can predict side effects of drugs with partially impressive accuracy
Within the project KI-NEW several classical modern Machine Learning methods as well as recent ones from the area of graph neural networks (relational graph convolutional and relational graph attention networks) have been modified and applied to the given problem. One of the specific challenges was the multi-modal nature of the partially high dimensional input data, which was addressed via a specific type of neural network architecture. Further challenges included the high implementation and computation demand within a very short time frame.
Based on available data KI-NEW demonstrated that AI approaches can predict side effects of drugs with partially impressive accuracy. Specifically, the results achieved via graph neural networks are scientifically interesting and innovative. They will contribute to a publication, which is currently in preparation. To finalize this publication some follow-up work needs to be completed, which e.g., addresses the explainability of achieved predictions. In parallel there are already ongoing discussions with industry partners.
 Research Center Machine Learning
Research Center Machine Learning